Federal Profile
Company Info:
DUNS: 062979000
CAGE: 0KDZ6
UEI: TVWAYN8M5D15
GSA: GS-07F-5355R
TYPE: SMALL BUSINESS
Product Codes:
NAICS Code
325620- Toiletry and Cosmetic Preparation Manufacture
325320- Pest Repellent
325412 – Pharmaceutical Preparation Manufacturing
424210- Drug and Cosmetic merchant wholesaler
UNSPSC CODE
10191500 – Pest Repellents
53131609 – Sun Protection
531316 – Personal Care Products
NIGP Codes:
48660 – Insecticides and Repellents, Household, Environmentally Certified Products
48500 – Janitor Supplies General
48513 – Hand Sanitizer – Hand and Skin
43600 – Germicides, Cleaners, And Related Sanitation Products for Health Care Personnel,
43673 – Soap, Surgical Scrub, Hand Sanitizer, Long-Term Killing Activity,
65232 – Dispensers for Personal Hygiene, Skin Care
A Few of our NSN Codes:
NSN: 8510-01-492-3313
Part: ICL-16-ESD
Static dissipative hand lotion
NSN: 8125-01-562-3875
Part: FD-2-ESD
ESD Safe Flux Bottle Applicator
NSN: 6850-01-394-0276
Part: ICSC-GAL
Workstation Surface Cleaner
NSN: 6850-01-429-9968
Part: TIP-T
Soldering Iron Tip Cleaner
Company Profile:
R&R Lotion is an American family-owned business established in Scottsdale, Arizona in 1983. Dedicated to Manufacturing new and innovated Formulations for the Bio Science, Electronic, Medical, Food Processing, Tactical, and Health & Safety Environment’s. Our United States Manufacturing plant is FDA/EPA/NSF Registered to Manufacture and Formulate OTC products.
Each year we reinvest a minimum of 10% of our Sales in R&D, Off Site Testing, New Lab, and Manufacturing Equipment to better serve our customers throughout the world. We reinvest in our employees with cGMP and OSHA training to further their education in the world of formulating and processing of products that we are producing.
Warehousing in Scottsdale, AZ and Rotterdam, Netherlands
U.S. Manufactured Tactical Products:
FDA/EPA/NSF REG. Quality Assured:
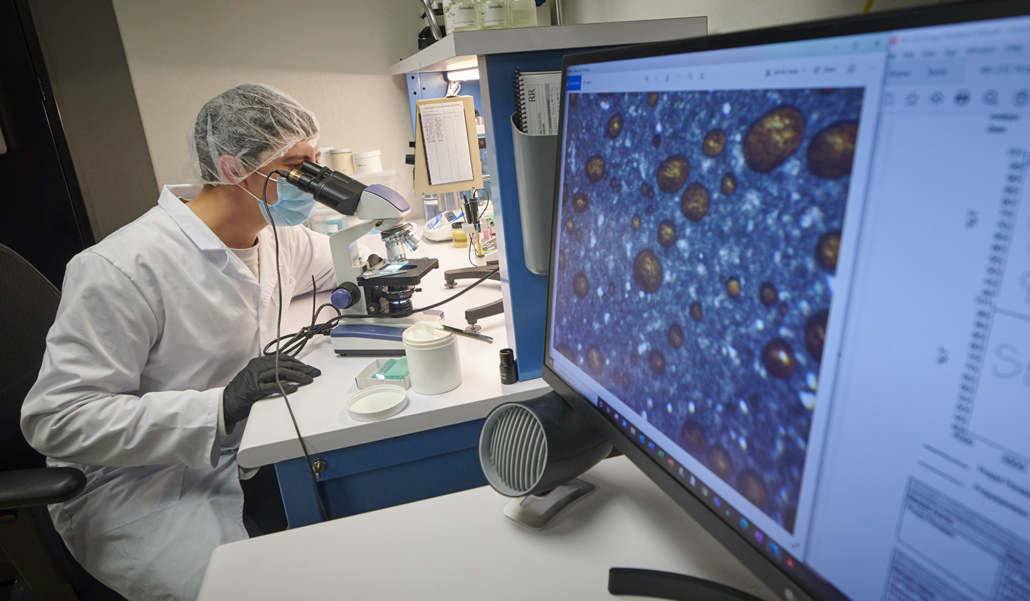
Our Cleaning process is adhered under FDA 21 CFR Part 211.67 for all our formulas whether they are FDA or EPA regulated. Our Production area, Tanks, Filling Equipment and Hoses are sanitized and tested after every use. R&R Lotion incorporates FDA 21 CFR 820.60 and 820.65 Identity and Traceability guidelines in case a recall is necessary.
All batches are sent out to independent labs to be tested for bacteria and active ingredients percentages before they can be released to our customers.
Research and Development:
For four decades we have been learning and asking our customers what they need and how we can help improve their lives. R&R Lotion prides itself with onsite staff to develop, formulate, and test new OTC products.